Caustic soda, also known as sodium hydroxide (NaOH), is an extremely versatile basic chemical extensively utilized across various industries. It is a powerful alkaline compound used in everything from manufacturing processes to household cleaning products.
This guide provides a comprehensive overview of caustic soda, covering its properties, forms, production methods, applications, safety, environmental impact, price, and much more.
Properties
The chart below summarizes the essential properties of caustic soda, providing a quick reference for its chemical and physical characteristics.
Property | Value |
Chemical Formula | NaOH |
Molecular Weight | 40.00 g/mol |
Appearance | White solid (in pure form) |
Melting Point | 318 °C (604 °F) |
Boiling Point | 1,388 °C (2,530 °F) |
Density | 2.13 g/cm³ (solid) |
Solubility in Water | 111 g/100 mL (20 °C) |
pH (1% solution) | ~14 |
Heat of Dissolution | -44.5 kJ/mol |
Specific Heat Capacity | 1.22 J/g·K |
Viscosity (aqueous solution) | Increases with concentration |
Conductivity (aqueous solution) | High |
Physical Forms
Caustic soda is available in 3 common physical forms: flakes, pearls (or beads), and liquid. Here’s a closer look at each form, including their pros and cons.
Caustic Soda Flakes

Caustic soda flakes are thin, flat pieces of solid sodium hydroxide. They are created by cooling and solidifying liquid sodium hydroxide and then breaking it into flakes.
Pros | Cons |
|
|
Caustic Soda Pearls (Beads)

Caustic soda pearls, also known as beads, are small, spherical pellets of solid sodium hydroxide. They are produced similarly to flakes but are shaped into small, round granules.
Pros | Cons |
|
|
Caustic Soda Liquid

This is a solution of sodium hydroxide in water. The concentration of the solution can vary depending on the intended application.
For industrial purposes, it is commonly available in concentrations ranging from 10% to 50% by weight.
Pros | Cons |
|
|
Production Methods

There are primarily two methods for producing caustic soda: causticization of soda ash and chloralkali process.
In terms of the design of the cells and the materials used to separate the anode and cathode compartments, the chloralkali process can be further divided into the mercury cell process, diaphragm cell process, and membrane cell process.
Each method has advantages and disadvantages regarding efficiency, cost, environmental impact, and product purity.
Pros | Cons |
|
|
Causticization of Soda Ash
This method involves the reaction between soda ash (sodium carbonate) and calcium hydroxide to produce caustic soda and calcium carbonate.
The chemical equation for this reaction is Na2CO3 + Ca(OH)2 → 2NaOH + CaCO3.
It is a relatively simple and traditional method of producing caustic soda, moreover, it is more environmentally friendly compared to other methods.
Pros | Cons |
|
|
Mercury Cell Process
Involves the electrolysis of brine (sodium chloride solution) using a mercury cathode and a graphite anode.
Sodium ions migrate towards the mercury cathode, where they react with liquid mercury to form sodium amalgam. Chlorine gas is produced at the anode due to chloride ions (Cl-) oxidation.
Sodium amalgam is then treated with water to release sodium hydroxide and regenerate the mercury, which can be reused in the process.
However, the mercury cell process has environmental concerns due to the use of mercury, and it has been largely phased out in many parts of the world.
Pros | Cons |
|
|
Diaphragm Cell Process
Similar to the mercury cell process, but use a porous diaphragm separates the cathode and anode compartments in the electrolytic cell.
Sodium ions migrate through the diaphragm towards the cathode where they react with water to form sodium hydroxide and hydrogen gas.
Chlorine gas is produced at the anode and does not mix with the sodium hydroxide solution, thanks to the diaphragm.
This process is more environmentally friendly than the mercury cell process but still has some environmental impact due to the production of chlorine gas.
Pros | Cons |
|
|
Membrane Cell Process
It’s an improvement over the diaphragm cell process, it uses an ion-selective membrane instead of a diaphragm to separate the anode and cathode compartments.
Sodium ions pass through the membrane towards the cathode, where they react with water to form sodium hydroxide and hydrogen gas.
Chlorine gas is produced at the anode and does not mix with the sodium hydroxide solution due to the selective membrane.
This process is more efficient and environmentally friendly than both the mercury cell and diaphragm cell processes, as it eliminates the use of mercury and reduces the production of chlorine gas.
Pros | Cons |
|
|
Applications
Caustic soda’s strong alkaline properties make it essential across a broad range of industries, from manufacturing and food processing to water treatment and energy production.
Here, we explore some of the typical applications of caustic soda and its critical role in modern industrial processes.
- Chemical Manufacturing: Caustic soda is fundamental in chemical manufacturing, serving as a strong base in various chemical reactions. It is used to produce a wide range of chemicals, including solvents, plastics, synthetic fibers, bleach, adhesives, coatings, herbicides, dyes, inks, and pharmaceuticals.
- Petroleum and Natural Gas Industry: Caustic soda is used for removing acidic contaminants from hydrocarbons and natural gas. It’s also used in drilling muds to increase mud viscosity and neutralize acidic gases, enhancing drilling efficiency and safety.
- Water Treatment: Caustic soda neutralizes acidic water, and precipitates heavy metals like lead and copper, facilitating their removal from the water supply. It is also used in desalination plants to regulate the pH and ensure efficient water treatment processes.
- Food Industry: In the food industry, caustic soda is used in various processes including peeling fruits and vegetables, processing cocoa and chocolate, thickening ice cream, and producing caramel color.
- Pulp and Paper Industry: Caustic soda helps break down lignin in wood chips during the digestion process, separating cellulose fibers for papermaking. In bleaching pulp, it is often used in combination with other chemicals like chlorine or hydrogen peroxide to remove color impurities and brighten the paper.
- Textile Industry: Caustic soda is used in the mercerization of cotton, a treatment that strengthens the fabric, enhances its dye uptake, and gives it a lustrous appearance. During the dyeing process, it helps to fix the dyes onto the fabric, resulting in vibrant and long-lasting colors.
- Aluminum Production: In the Bayer process for aluminum ore (bauxite) refining, caustic soda is used to dissolve aluminum-bearing minerals, separating them from impurities such as silica and iron oxides. In the Hall-Héroult process, caustic soda serves as an electrolyte in the electrolytic cells where aluminum is produced from alumina through electrolysis.
- Soap and Detergent Manufacturing: Caustic soda facilitates saponification, converting fats/oils into soap. It adjusts pH levels, ensuring product stability and enhances cleansing properties. Additionally, it aids in neutralizing acids and controlling viscosity.
Safety and Handling
Handling caustic soda (sodium hydroxide) requires careful attention to safety procedures due to its corrosive nature. Here are some guidelines for personal protective equipment (PPE), storage, mixing and dilution, first aid measures, and spill and leak response.
Personal Protective Equipment (PPE)
- Protective Clothing: Wear chemical-resistant clothing made from materials such as PVC, neoprene, or other alkali-resistant fabrics. Ensure the clothing covers all exposed skin, including long sleeves and full-length pants.
- Gloves: Use gloves made from rubber, PVC, neoprene, or nitrile. Make sure the gloves cover the wrists and extend up the arms for added protection.
- Eye Protection: For added protection, use a face shield in combination with goggles, especially when handling large quantities.
- Respiratory Protection Type: When handling caustic soda dust or aerosols, use a respirator with appropriate cartridges (e.g., N95 or P100).
- Foot Protection: Wear chemical-resistant PVC or rubber boots. Boots should have non-slip soles and be high enough to cover the ankles and prevent splashes from entering.
Storage
Proper storage is essential to ensure safety and maintain the caustic soda’s quality.
- Storage Containers Material: Use containers made of materials resistant to caustic soda, such as carbon steel, stainless steel, polyethylene, or polypropylene.
- Environment Conditions: Store in a dry area with good ventilation, and ensure it has a temperature between 10°C and 30°C (50°F and 86°F) to prevent moisture absorption, crystallization or degradation.
Transportation

- Transportation Modes: Transportation Modes Caustic soda can be transported via various modes, including road, rail, and sea.
- Regulatory Organizations: Transporting caustic soda involves adhering to regulations set forth by several regulatory organizations (such as DOT, IMO, ECHA, and OSHA). These organizations provide guidelines for the safe handling, packaging, labeling, and transportation of hazardous materials, including caustic soda.
First Aid Measures
In case of exposure, following specific first aid measures is crucial. Here are the recommended first-aid measures for skin contact, eye contact, and inhalation of caustic soda:
- Skin Contact: Immediately remove any clothing that has come into contact with caustic soda. Flush the affected area with plenty of lukewarm water for at least 20 minutes. If irritation persists, if there is significant pain, or if the exposure is extensive, seek medical attention immediately.
- Eye Contact: Hold the eyelids open and flush the eyes with lukewarm water continuously for at least 20 minutes. Use an eye wash station if available. After the flushing, get medical help immediately.
- Inhalation: Immediately move the person to a fresh air area. Check the person’s breathing, if they are having difficulty breathing, provide oxygen if available(avoid mouth-to-mouth resuscitation). Call emergency services immediately and seek professional medical help.
Spill and Leak Response
Prompt and proper response to spills and leaks is vital to prevent injury and environmental damage.
- Containment: Isolate the area and prevent the spread of the spill. Use barriers or absorbent materials to contain the liquid.
- Neutralization: Apply a neutralizing agent, such as diluted acetic acid or vinegar, to the spill, following the manufacturer’s instructions.
Environmental Impacts & Solutions
Despite its widespread utility, caustic soda’s production and usage can have significant environmental impacts that necessitate attention and solutions.
- Waste Generation: The manufacturing process generates waste products, including brine sludge and spent caustic soda solutions requiring proper disposal.
- Water Pollution: Improper disposal of caustic soda or its byproducts can lead to water pollution, affecting aquatic ecosystems. Additionally, accidental spills can contaminate water sources.
- Air Pollution: During caustic soda production, emissions of pollutants such as chlorine gas and mercury may occur.
Implementing stringent environmental regulations and pollution control measures can help prevent water and air pollution associated with caustic soda manufacturing.
Adopting closed-loop systems and advanced neutralized wastewater treatment technologies can help minimize the generation of hazardous waste and pollutants from caustic soda production.
Additionally, recycling caustic soda solutions and by-products can reduce resource consumption and promote circular economy principles.
Why is Caustic Soda So Expensive?
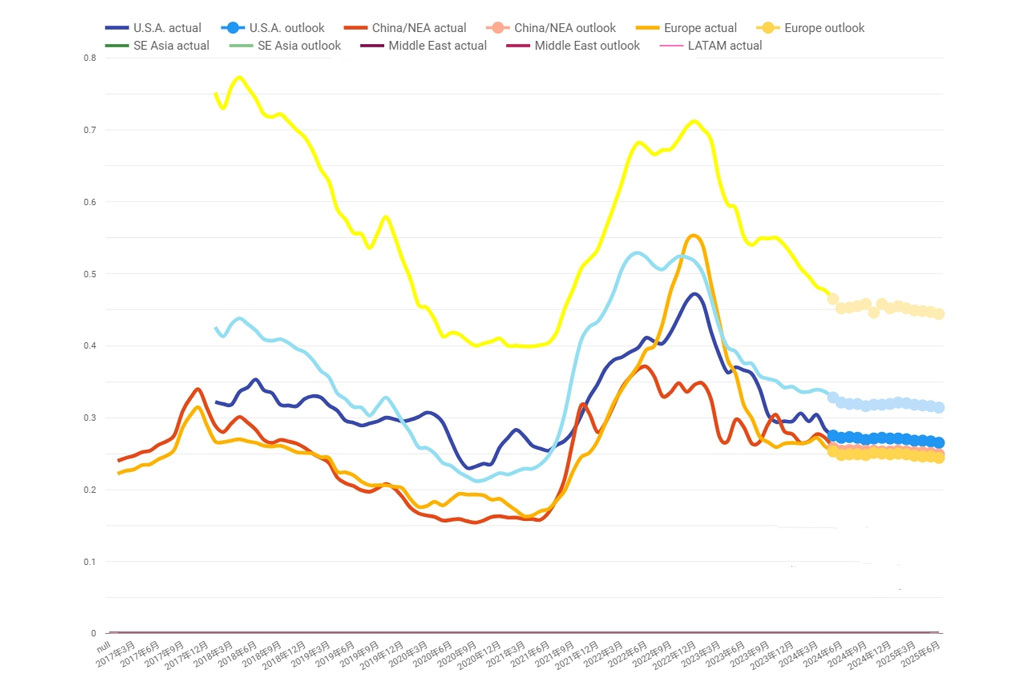
Recently, the cost of caustic soda has surged significantly, prompting industries reliant on this chemical to reevaluate their budgets and procurement strategies.
In the year of 2024, the average price of caustic soda is around $0.25 USD per kilogram.
Factors That Impact The Price?
- Raw Materials: The primary raw material for caustic soda production is common salt (sodium chloride). Fluctuations in salt prices due to factors like demand, supply, and transportation costs directly affect caustic soda prices.
- Purity Level: Higher purity caustic soda has a higher price due to the additional processing and quality control required to remove impurities.
- Energy Costs: Caustic soda production involves an energy-intensive process known as electrolysis, where an electric current passes through a saltwater solution to produce sodium hydroxide. Any increase in energy prices can elevate the overall production cost of caustic soda.
- Regulatory Compliance: Stringent environmental regulations necessitate investments in pollution control measures and adherence to safety standards, adding to the production expenses. Compliance with these regulations can result in higher prices for caustic soda.
- Market Demand: The demand for caustic soda fluctuates across industries, leading to variations in its price.
- Transportation Costs: Caustic soda is often transported in bulk quantities, which incur transportation expenses. Factors like fuel prices, distance, and infrastructure can influence transportation costs, subsequently impacting the product’s final price.
Where to Buy Caustic Soda at A Cheap Price?
- Direct from Manufacturers: Contacting caustic soda manufacturer (especially from China) directly can often lead to cost-effective procurement options. China is currently the world’s largest producer of caustic soda, followed by the United States and Europe.
- Distributors: Wholesale distributors specializing in industrial chemicals often offer competitive prices. They typically have established networks with multiple manufacturers, allowing them to source products at favorable rates.
- Group Purchasing Organizations (GPOs): Joining a GPO, consisting of multiple businesses pooling their purchasing power, can yield significant cost savings on caustic soda and other industrial supplies.
- Online Marketplaces: E-commerce platforms have transformed the way businesses procure caustic soda.

